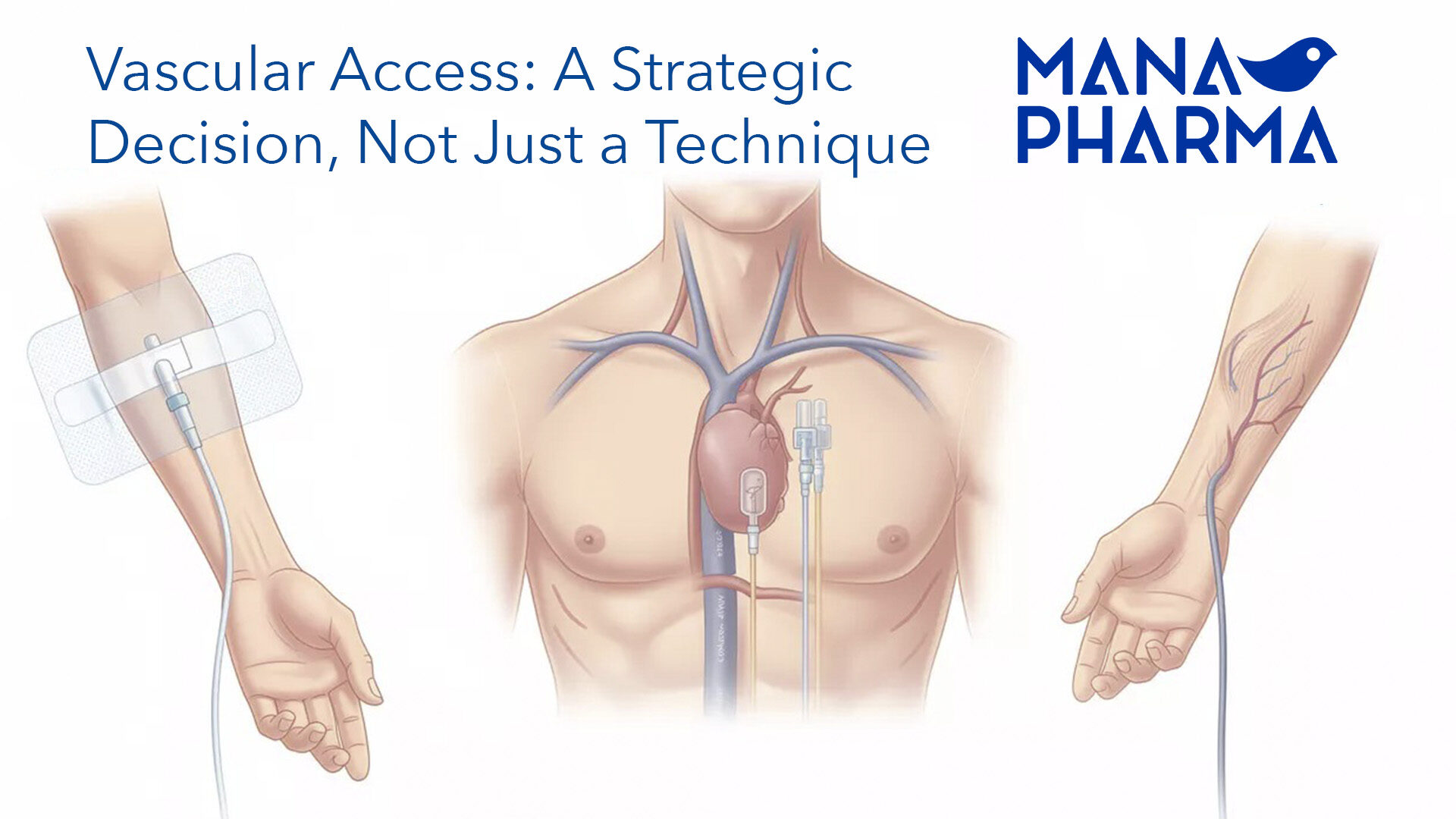
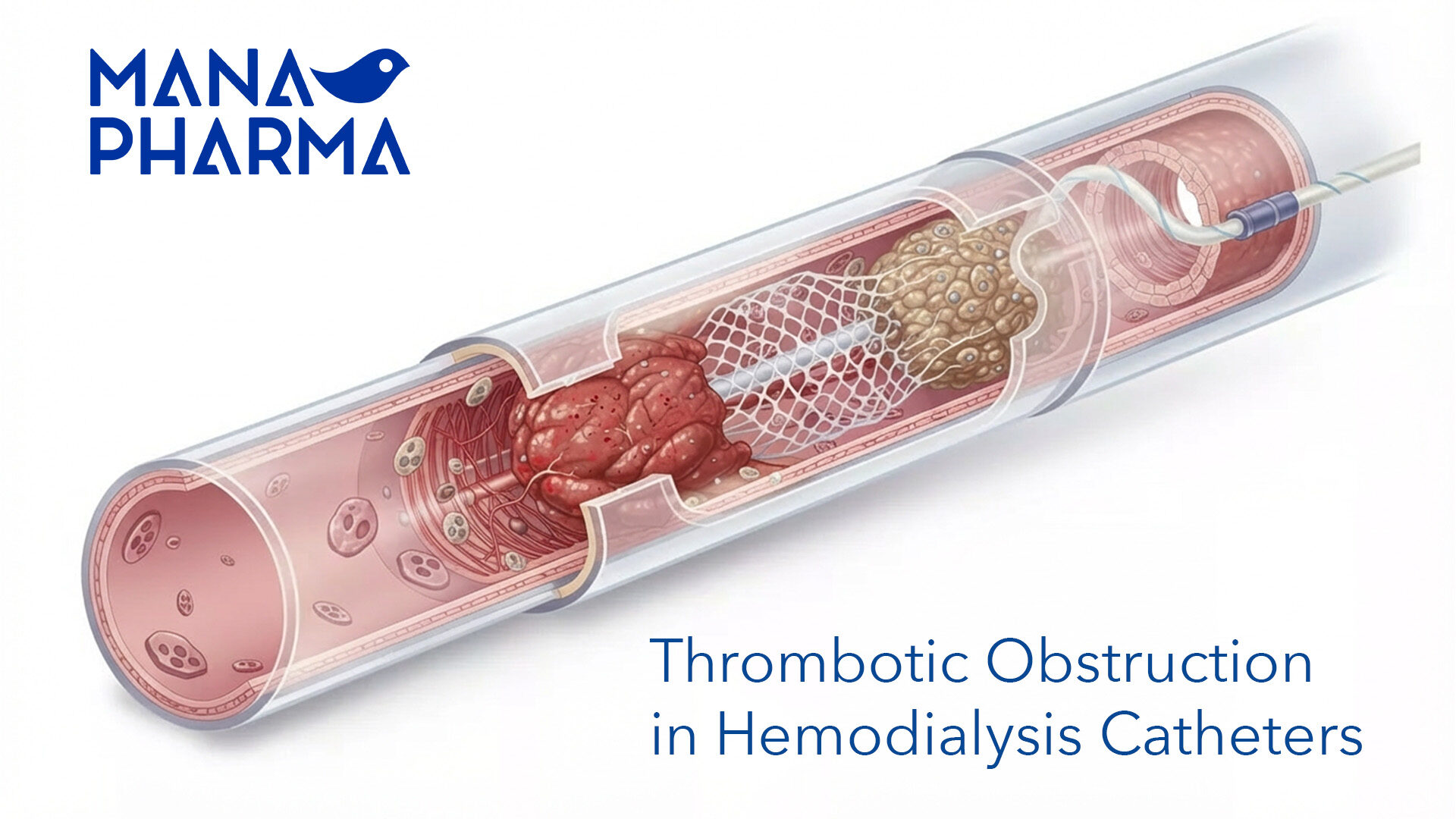
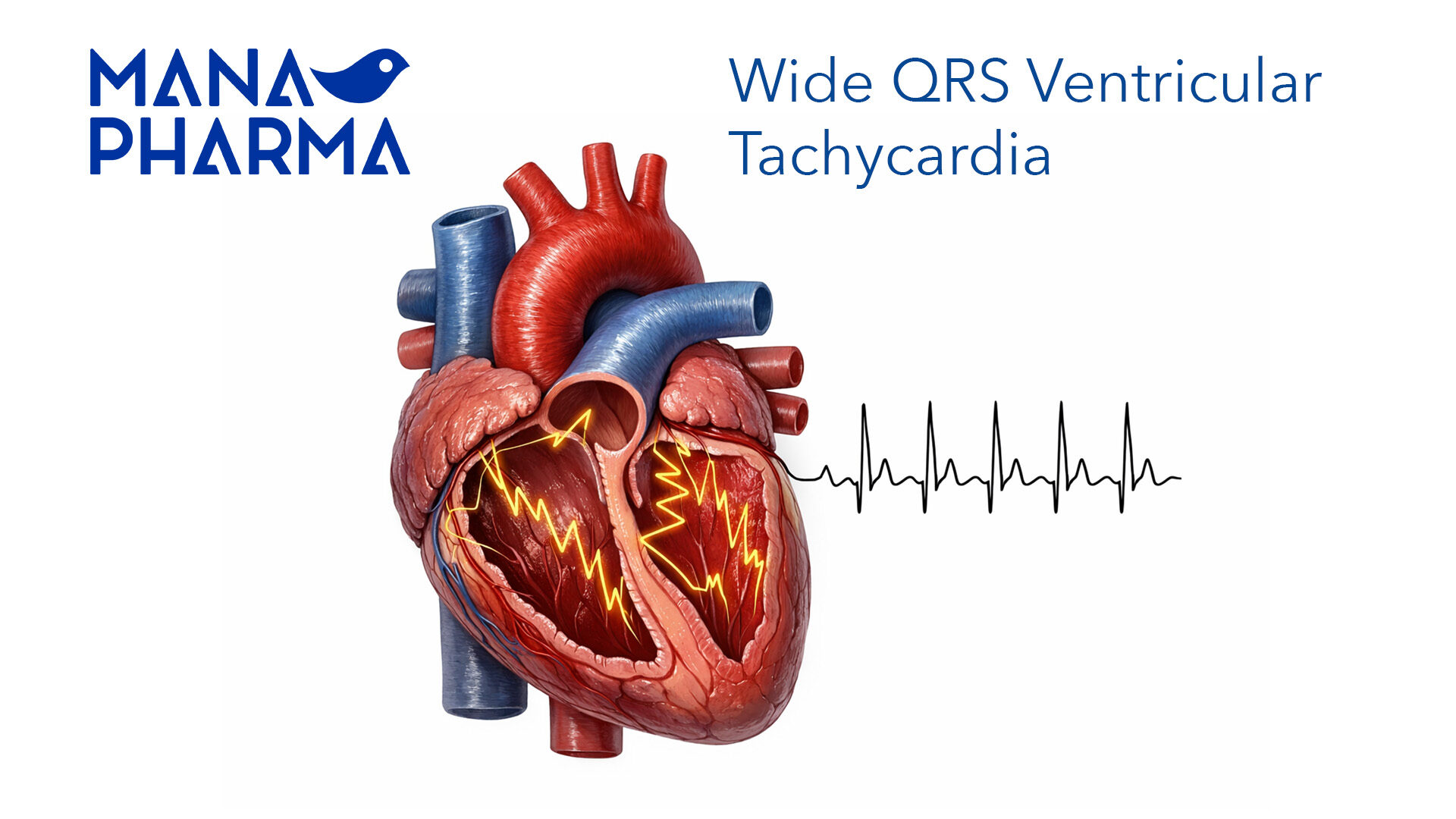
Our team efficiently and accurately manages the registration process for the wide range of products aforementioned, ensuring full compliance with both national and community regulations for each one of the target countries. Additionally, they carefully oversee the lifecycle of medications, managing renewals and variations promptly for all products involved, thus guaranteeing that the continuity and quality of their supply.
Our Regulatory Affairs Department facilitates access to international markets through a comprehensive “turnkey” approach, guiding our clients through the complex regulatory requirements of each country. We specialise in document management and communication with Regulatory Agencies, responding as soon as possible to their inquiries and maintaining fluid and transparent communication throughout the entire registration process.
Our commitment to excellence is reflected in conducting rigorous audits of registration dossiers to ensure accuracy and compliance with applicable regulations, thereby minimising regulatory risks and ensuring the quality of the documentation submitted before the registration process has even started. In addition, internally, we follow a Regulatory Intelligence strategy that allows us to keep abreast of evolving regulatory changes in the markets in which we operate, ensuring that our clients are always informed and the dossiers we submit to the relevant Regulatory Agencies are in full compliance with the latest regulations in this field.
If you have any regulatory needs in non-EU markets, do not hesitate to contact us.