Temperature Control for Thermolabile Medicines

Contingency Plan: Ensuring Cold Chain Preservation During Power Outages One thing we know for sure is the importance of maintaining the correct temperature for thermolabile
What is a Marketing Authorization Holder (MAH) Laboratory?

In the pharmaceutical sector, the distribution and marketing of medicines are subject to strict regulations to ensure drug quality, safety, and efficacy. One of the
What are Medicines under Special Situations (MSS)?

Ensuring Supply Amid Shortages In the Spanish pharmaceutical sector, one of the biggest challenges is the shortage of essential medicines, a situation that particularly affects
World Immunization Week 2025
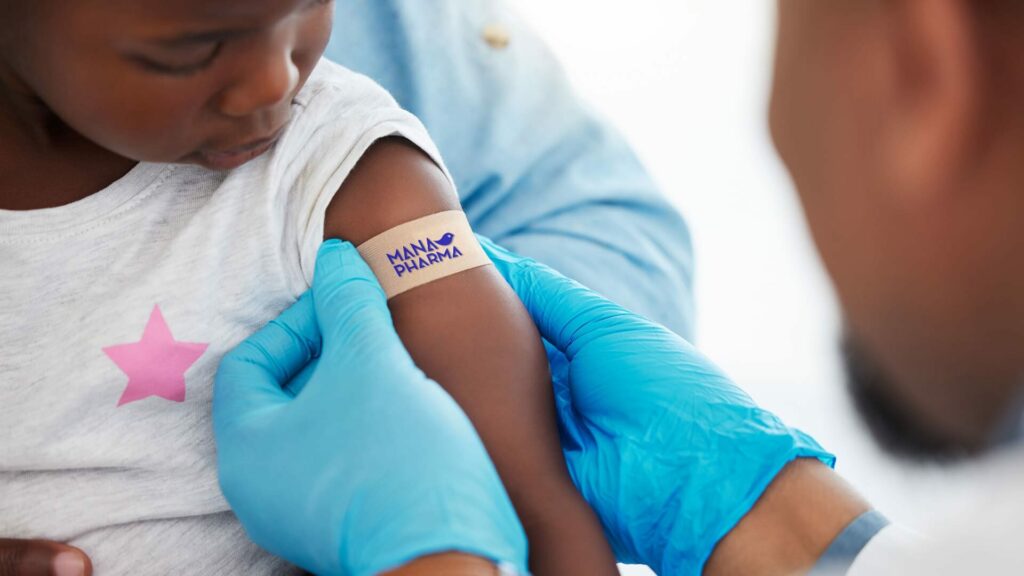
Every year, the World Health Organization (WHO) reminds us of something fundamental: vaccines save lives. In 2025, the theme is: “Immunization for all is humanly
Characteristics of an efficient pharmaceutical warehouse and cost-effective logistics strategies

The storage of pharmaceutical products is a critical process that requires specialized infrastructure, strict controls, and efficient logistical strategies. A well-functioning warehouse must ensure the optimal preservation of medicines and other healthcare products, while complying with all regulatory requirements and quality standards.
The Reform of Pharmaceutical Legislation in the EU: A Step Towards Innovation and Equity in the Single Market

The Reform of Pharmaceutical Legislation in the EU: A Step Towards Innovation and Equity in the Single Market
Drug serialization system: Advances and challenges

The counterfeiting of medications poses a direct threat to the health of millions of people each year. In response to this ever-growing threat, the European Union decided to intensify its efforts by adopting stricter regulatory measures that culminated in 2019 with the implementation of Directive 2011/62/EU and Delegated Regulation (EU) 2016/161.
MANA PHARMA: Committed to Supplying Medicines to Hospitals in Spain

Over the past few years, the Healthcare System in Spain has faced increasing challenges in ensuring a reliable supply of essential medicines. The shortage of certain drugs, a pressing issue impacting both patients and Healthcare professionals, has underscored the importance of a robust and reliable Distribution System.
MANA PHARMA IN CHINA

At Mana Pharma, S.L., we have taken a significant new step as part of our international expansion strategy: we have signed a collaborative agreement with our partner in China, from the Anhui region near Beijing. This milestone strengthens our presence in the Asian market and reinforces our commitment to bringing health to all corners of the world.
Importation of medical devices

In order to guarantee the supply and quality of Medical Devices in the Health System, MANA PHARMA, S.L., as an Importer of Medical Devices, carries out the following activities…





