1) IMPORTATION ACTIVITY OF MEDICAL DEVICES (IPS)
In order to guarantee the supply and quality of Medical Devices in the Health System, MANA PHARMA, S.L., as an Importer of Medical Devices, carries out the following activities:
- Researches the users’ and patients’ needs by following up on official tenders and direct contact with Customers.
- Selection of products with outstanding quality and technology.
- Studies and evaluates the technical documents of the product and the product manufacturer according to the Technical-Sanitary criteria.
- Import of the product.
- Inspection and Release of the imported product.
- Distribution of the product with guaranteed quality.
- Post-market monitoring and surveillance of the safety of the use.
Why is the Importer´s role important in Medical Devices?
The Importer of Medical Devices is responsible for the introduction of a device manufactured in a third country into the country of commercialization. Therefore, it must guarantee that the product´s quality and characteristics comply with the regulations of the country of commercialization.
On the other hand, the Medical Device Importer must follow up on possible claims, queries, incidents, etc… related to the imported Medical Device.
What kind of medical devices do we import?
MANA PHARMA, S.L. is officially authorized by the Spanish Agency of Medicines and Medical Devices.(AEMPS) to import and be an Authorized Representative of:
– HEALTH CARE PRODUCTS CLASS I, IIa, IIb AND III (INCLUDING ACTIVE IMPLANTABLE PRODUCTS).
- Healing materials: dressings, absorbents, bandages, etc…
- Fluid administration equipment: syringes, infusion sets, nutrition material, etc….
- Hygiene and protection: gowns, gloves, masks, surgical drapes, incontinence material, etc…
- Lancing and incision material: needles, trocars, cannulas and catheters, scalpels, etc….
- Samples and waste containers: tubes, collection bags, specimen collection bottles, drainage collectors, etc….
- Suture material: suture needles, manual suture, mechanical suture.
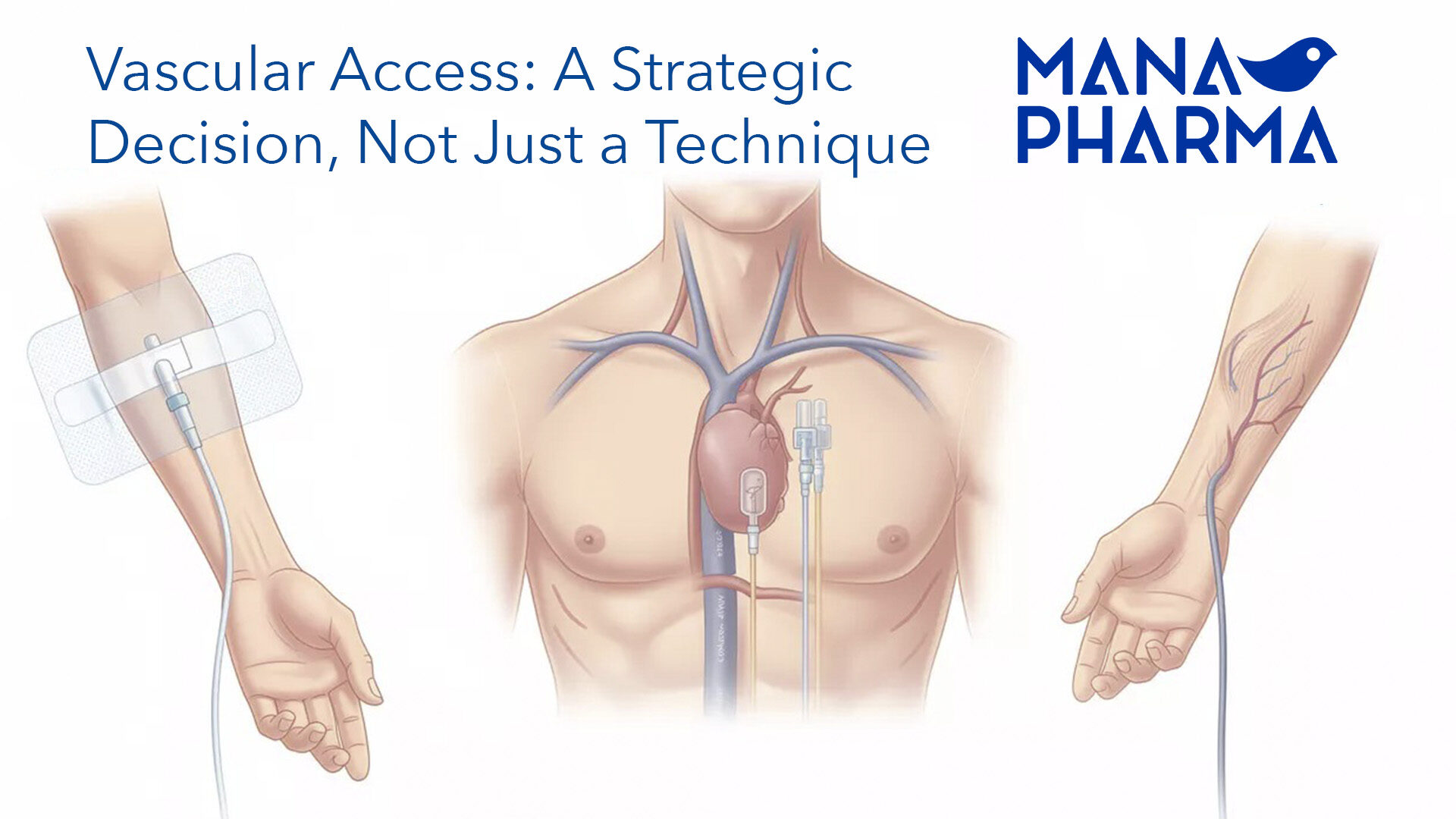
- Cannulas, catheters, probes, tubes: tracheotomy, suction, urethral, gastric, nutrition, endotracheal, etc…
- Oxygen therapy, aerosol therapy and assisted ventilation equipment: masks, nebulizers, filters, ventilation circuits, humidification systems, etc…
- Hemostatics in general
- Medical Software.
- Medical devices containing medicinal substances.
- Active Implantable Products.
- Non Active Implants.
- Other medical devices mentioned in the Manual “CCPS Marketing and/or Commissioning Communications for Medical Devices Product Categories, Generics and Subgenerics according to MDR and IVDR) Version 2.0.1. n the Manual “CCPS Marketing and/or Commissioning Communications for Medical Devices Product Categories, Generics and Subgenerics according to MDR and IVDR) Version 2.0.1.
– IN VITRO DIAGNOSTIC MEDICAL DEVICES
2.a. Classification Under Regulation (EU) 2017/746 of the European Parliament and of the Council. 4321
- Class A devices
- Products for general laboratory use
- Class B devices
- Class C Products
- Class D Products
2.b. Classification under Royal Decree 1662/2000, of September 29, 2000, on medical devices for “in vitro” diagnosis.
- Annex II List A Products
- Annex II List B Products
- Self-diagnostic products
– Non-Medical Devices referred to in Annex XVI of Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices.
1. Contact lenses or other articles intended to be inserted or placed in the eye.
2. Products intended to be wholly or partially introduced into the human body by invasive means of a surgical type for the purpose of modification of the anatomy or fixation of parts of the body, with the exception of products for tattooing and piercing.
3. Substances, combinations of substances or articles intended for use as facial or other dermal or mucous membrane fillers by subcutaneous, submucosal or intradermal injection or by other means of introduction, excluding those intended for tattooing.
4. Equipment intended to be used to reduce, remove or destroy adipose tissue, such as equipment for liposuction, lipolysis or lipoplasty.
5. Equipment emitting high intensity electromagnetic radiation (e.g., infrared, visible and ultraviolet light) intended for use on the human body, including coherent and non-coherent, broad spectrum monochromatic sources, such as lasers and intense pulsed light equipment for skin rejuvenation, tattoo removal, hair removal or other dermal treatments.
6. Equipment for brain stimulation that applies electric currents or magnetic or electromagnetic fields that penetrate the skull to modify the neuronal activity of the brain.
– Other Products regulated by the Medical Devices Legislation
Devices and instruments used for permanent or semi-permanent make-up or tattooing of the skin by invasive techniques, not considered Medical Devices.
2) AUTHORIZED REPRESENTATIVE ACTIVITY
Authorized Representative is a regulatory service that only applies to Medical Devices. All manufacturers located in non-EU countries must hire and officially appoint an Authorized Representative in the EU in order to be able to market the products they manufacture.
In the IPS activity (Importation of Medical Devices) MANA PHARMA, S.L. distinguishes itself in the industry in the following critical aspects:
1) Having Head Office in Europe and official offices in Africa, Central and South America causes the management and wider service and closer relationship with Suppliers, Customers and Authorities. It also facilitates order management and improves communication.
2) Wide scope of the activity: The scope of the IPS activity allows us to have the possibility to develop and collaborate in different projects with the manufacturers.
MANA PHARMA, S.L. is one of the few companies Importing Medical Devices that is authorized by the AEMPS to import all types of Medical Devices, Medical Devices for In Vitro Diagnostics and Non-Medical Devices for aesthetic uses.
3) MANA PHARMA, S.L. facilitates the introduction of Medical Devices manufactured outside the European Union into the European market through the activity of Authorized Representative.
4) ISO Certificates: MANA PHARMA endorses the high quality standards by obtaining the Certificates:
-
- EN ISO 13485:2016/ A11:2021
- ISO 9001:2015
with scope to provide Import, Warehousing, Distribution, Export and Authorized Representation Services for Europe, for:
– Medical devices:
- Non-active and active implantable medical devices in general.
- Active and non-active Medical Devices in general.
- In Vitro Diagnostic Medical Devices:
- Reagents and reagent products
- Calibrators and control materials in general
- IVD medical devices in general
- Medical devices without medical purpose according to current regulations
- Devices for Tattooing, Permanent and Semipermanent Make-up
5) The quality and service provided by MANA PHARMA, S.L. are guaranteed by more than 30 official Certificates and Authorizations, which demonstrate the confidence of the Health and other Authorities in the Quality Management Systems implemented in MANA PHARMA, S.L.
6) Multidimensional Services
MANA PHARMA, S.L. is part of NET PHARMA HUB, a pharmaceutical cluster formed by more than 30 pharmaceutical companies with different activities, which establish synergies and active and continuous collaborations. Therefore, both MANA PHARMA, S.L. and other members of NET PHARMA HUB, can offer customers a comprehensive service and meet their needs and requirements, such as import, export, distribution, storage, release, calibration, validation, etc…