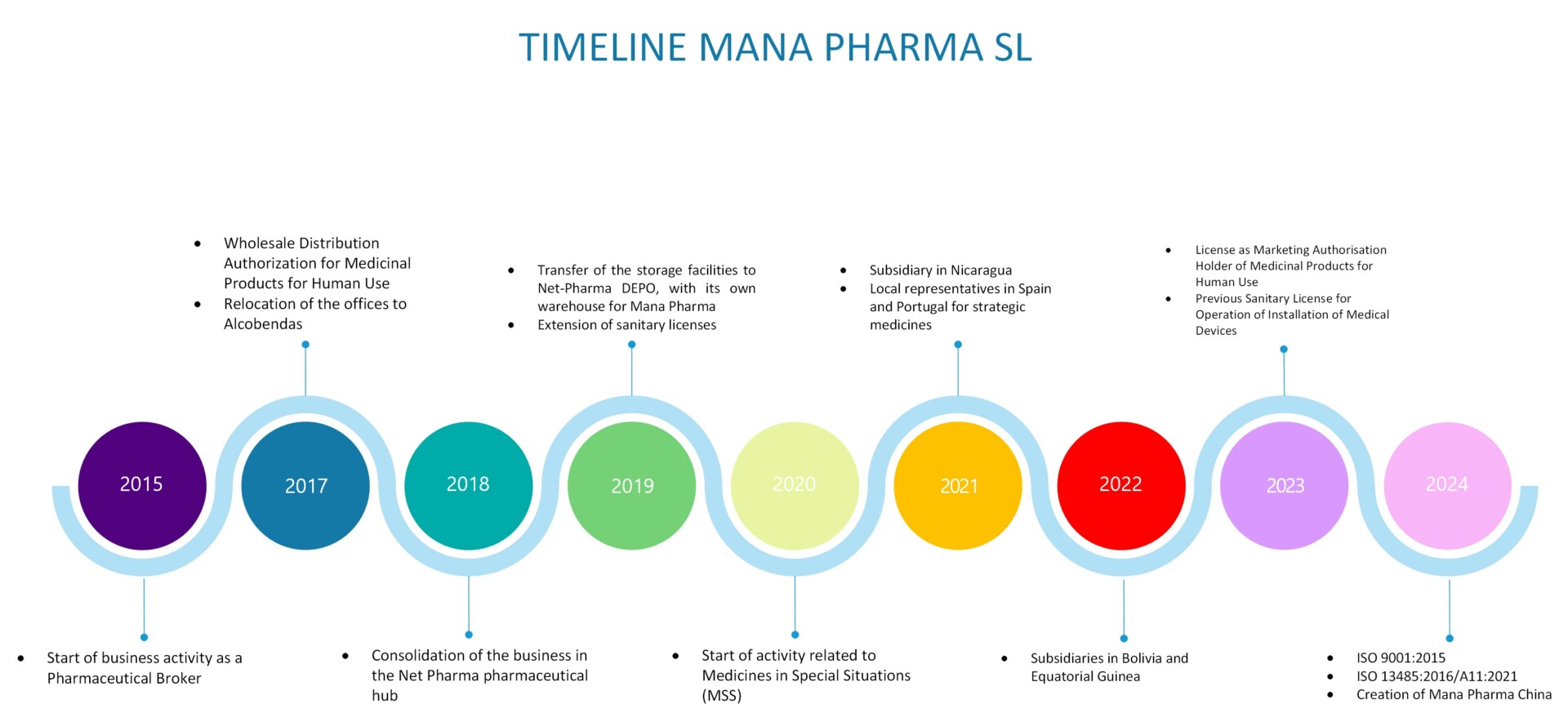
In May 2017, MANA PHARMA S.L. obtained authorization as a wholesale distributor of pharmaceutical products for human use, providing the organization with the necessary infrastructure to obtain, store, distribute, and export medicines and pharmaceutical products in general. This development allowed for greater versatility in the business operations, expanding the portfolio of products and services, strengthening relationships with existing clients and suppliers, establishing new partnerships, and expanding into the international market.
In November 2017, the company’s headquarters were established in Alcobendas, where the current offices are located within the NET-PHARMA pharmaceutical hub. This allowed MANA PHARMA S.L to consolidate its business throughout 2018 and position itself in the sector, establishing synergies with other pharmaceutical companies.
In November 2018, NET-PHARMA DEPO S.L. was created thanks to the investment of MANA PHARMA S.L. In July 2019, this company obtained authorization as a wholesale distributor of pharmaceutical products for human use with contract warehouse activity, at which point MANA PHARMA S.L. decided to relocate its storage facilities to this location, thereby gaining its own warehouse.
This led to the updating of the healthcare licenses held by MANA PHARMA S.L. and the acquisition of new authorizations, registrations, and certifications, expanding the scope of activity to APIs (Active Pharmaceutical Ingredients), excipients, medical devices, dietary supplements, chemicals, industrial and hospital equipment, and the provision of various pharmaceutical services (registration of medicines in countries inside and outside the EU, consulting services for the development of Quality Management Systems, obtaining various healthcare authorizations and licenses for pharmaceutical companies or for those wishing to enter this sector, construction of pharmaceutical plants and hospitals with equipment supply, participation in national and international tenders, etc.).
It is worth highlighting the significant healthcare activities carried out by MANA PHARMA S.L. within the national territory, initiated at the end of 2020, related to Medicines in special situations (MSS) in collaboration with healthcare authorities, thus helping to alleviate the shortage of medicines in our country. Additionally, MANA PHARMA S.L. distributes medicines in Spain from European MAH laboratories that have placed their trust in us, with MANA PHARMA S.L. taking on the role of Local Representative before the Spanish healthcare authority.
As a result, in 2021, MANA PHARMA S.L. became the Local Representative in Spain and Portugal for strategic medicines from a European MAH laboratory.
In adition, during 2021 and 2022, subsidiaries were opened in Nicaragua, Bolivia, and Equatorial Guinea, which are key points on the international front, although it was not until 2023 that the corresponding healthcare authorizations were obtained.
Following positive feedback from both national and international activities, with the expansion in the market and the broadening of the supplier and client portfolio, MANA PHARMA S.L. obtained, in June 2023, the Lincese as Marketing Authorisation Holder (MAH) of Medicinal Products for Human Use. This new and significant achievement provides the organization with an additional link in the supply chain of medicines and represents a further step forward by having MANA PHARMA S.L. branded medicines for human use.
Another important milestone in the trajectory of MANA PHARMA S.L. took place in January 2023 with the acquisition of the Previous Sanitary License (conditional) of operation for Installation of Medical Devices (Activity: Importation of Medical Devices), the scope of which was modified in December 2023.
As the culmination of its medical device importation activities, in July 2024, the company obtained the EN ISO 13485:2016/A11:2021 Certification as well as the ISO 9001:2015 Certification.
In July 2024, MANA PHARMA China offices were opened.
Our goal is to generate and maintain strong relationships with our clients based on trust, offering quality service at competitive prices. To achieve this, we are commited to complying with all legal and regulatory requirements, as well as the requirements from our clients and suppliers, or those we have internally agreed upon.
This would not be possible without our valuable team of qualified professionals who provide personalized service to our clients, considering not only their current needs but also anticipating their future expectations, fostering communication, and creating the necessary structure for continuous improvement.
Our commitment extends to our suppliers, who are selected and evaluated in terms of their service and quality to offer our clients the highest guarantee.
We continue to make progress in the consolidation and growth of the company, facing new challenges that will soon bear fruit.